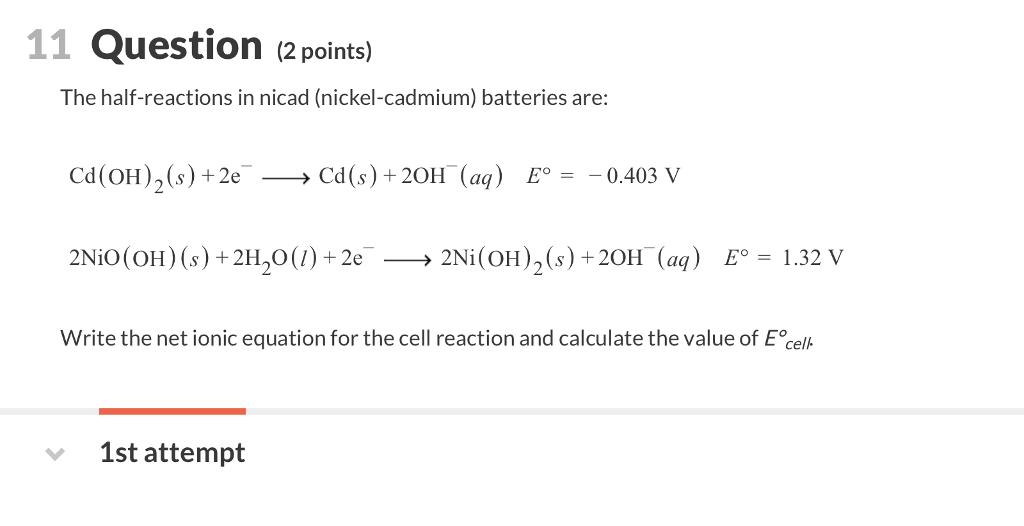
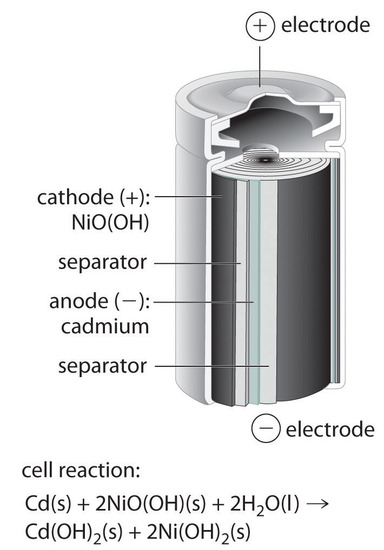
Nickel cadmium battery the nickel cadmium nicd battery is another common secondary battery that is suited for low temperature conditions with a long shelf life.
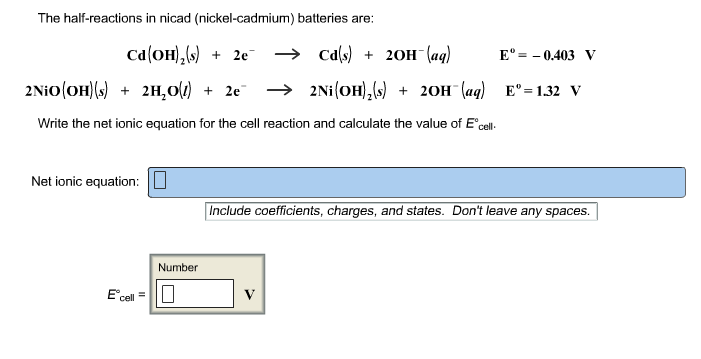
Nickel cadmium battery equation.
Nickel cadmium battery redox reactions and electrochemistry chemistry khan academy.
In eqns 4 6 the cell reactions during charging and discharging are presented.
However the robust nicd batteries are very durable reliable easy to use and economical.
Nickel cadmium batteries at the 1 2h rate yield twice the energy density of lead acid batteries i e.
Our mission is to provide a free world class education to anyone anywhere.
So the nickel cadmium battery is like the lead storage battery it s rechargeable and therefore it can be very useful.
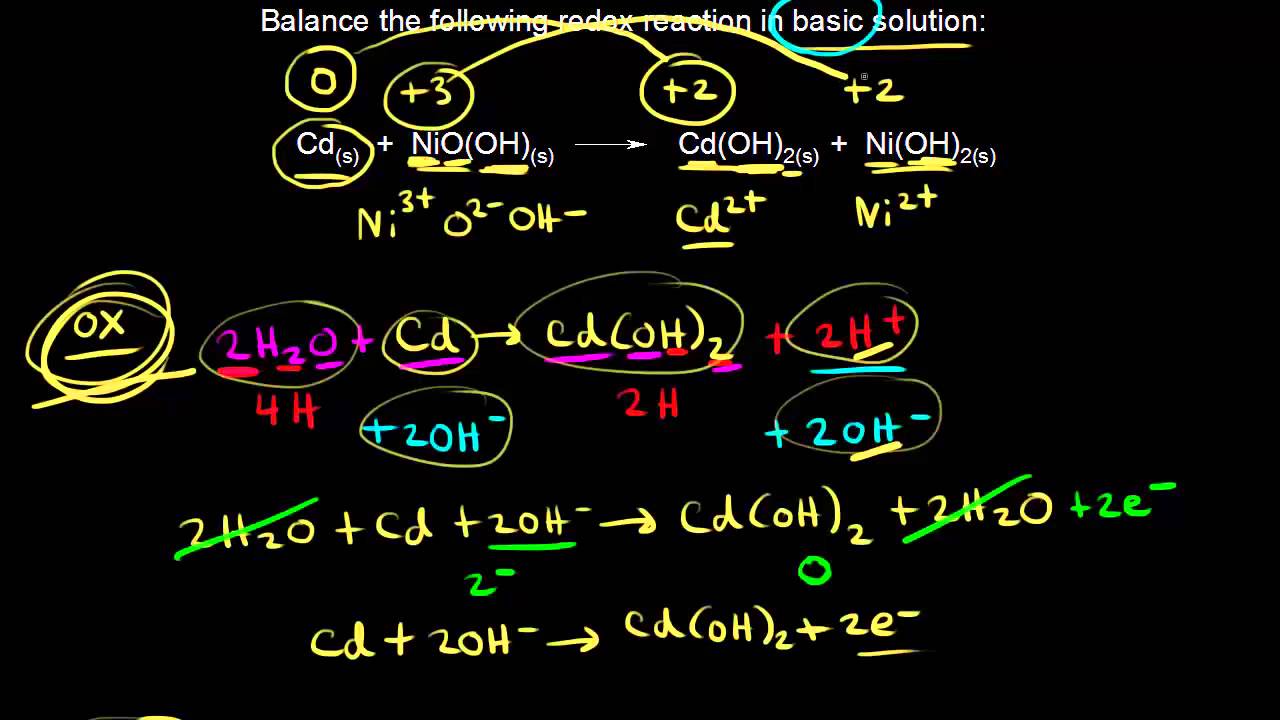
Nickle cadmium battery is a rechargeable battery with nickle oxide hydroxide and cadmium as electrodes and alkaline potassium hydroxide as electrolyte.
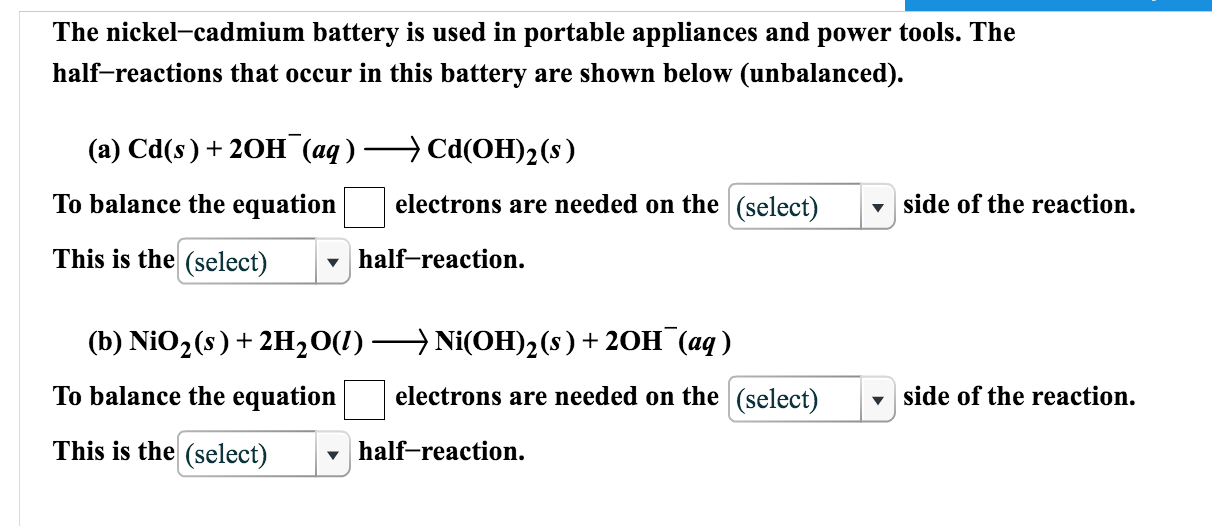
The nickel cadmium battery ni cd battery or nicad battery is a type of rechargeable battery using nickel oxide hydroxide and metallic cadmium as electrodes.
Battery amp hour.
Nickle cadmium battery was invented by swedish inventor waldemar jungner in 1899 and popularized and widely manufactured only during 1940 s and 1950 s.
The maximum cell voltage during charge is 1 3 v and the average cell voltage is 1 2 v.
The nickel cadmium battery system still uses the same positive electrode as the nickel iron one while the negative electrode is cadmium.
Nickel cadmium nicd is one of the most established amongst the various commercially available rechargeable battery systems.
Nernst equation redox reactions and electrochemistry.
Nickel cadmium batteries are used in various developmental electric vehicles.
The energy density of nicd batteries are lower than the newer battery systems such as nickel metal hydride and lithium ion.
They operate over a wide temperature range give approximately 2000 cycles and can be charged in less than 1 h.
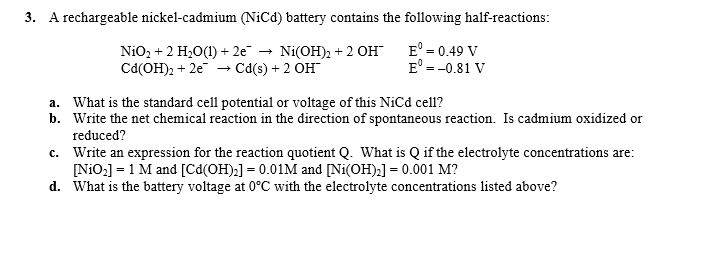
2015 ap chemistry free response 1d.